

| | Nowadays, it is practically impossible for health professionals to practice without the aid of technology. However, technology is nothing but a tool; as such, it must be used skillfully and wisely to generate the expected benefits, while carefully avoiding its pitfalls and risks as much as possible. Many of the "medical errors" have been blamed on clinicians with little consideration to the facts that many technologies were improperly design, selected, or supported. For example, few administrators are aware of the fact that technology costs are mostly "buried under the ocean" as graphically demonstrated below. 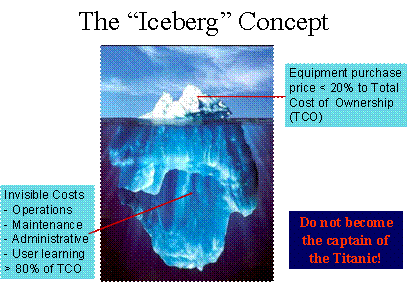
Because of the vast experience of its talented consultants, BSI is uniquely positioned to assist technology users to make the right decision for their particular environment in every part of the technology management life cycle. 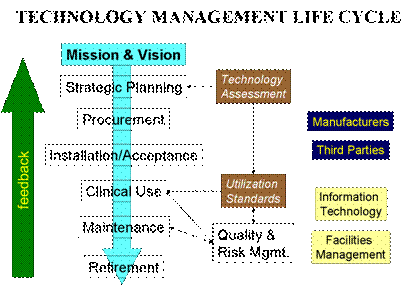
 | Strategic planning  | strategic analysis (strength, weakness, opportunities and threats - SWOT) |  | safety, efficacy and effectiveness assessment reviews |  | potential risks and liabilities exposures evaluations |  | needs assessment (requirements to meet organization mission, vision, goals and objectives) |  | impacts assessment (infrastructure, users, and technical support) |  | cost assessment (total cost of ownership analysis) |  | benefits assessment (clinical, financial, strategic, etc.) |  | return on investment analysis |
|
 | Procurement | technology evaluation |  | product evaluation |  | selection process (bidding, request for proposal, direct negotiation, etc.) |  | purchase agreement |  | alternatives to purchasing (leasing, rental, revenue sharing, etc.) |
|
 | Installation & Acceptance | pre-installation requirements |  | installation verification and validation |  | acceptance testing |  | notification to respective OEM, if required |
|  | Clinical use | user training/inservice and retraining |  | clinical protocol |  | use error monitoring |
|  | Maintenance | servicer training |  | warranty management |  | safety and performance inspections and preventive maintenance |  | revision control |  | service options (internal, OEM, third parties) |  | service records |  | test & measurement equipment calibration |  | service contract review and supervision |
|  | Retirement | retirement and replacement planning |  | replacement criteria |  | notification to respective OEM, if required |  | remarketing or donation |
|  | Quality and Risk Management | FDA general controls against misbranding and adulteration |  | recall program |  | device tracking program |  | complaints management |  | sourcing of alternative parts and subassemblies |  | patient/user incident investigation |  | liability insurance |  | litigation defense |  | compliance to voluntary standards (JCAHO, ISO 9001:2000, etc.) |
|  | Interface | with facility management |  | with information technology |  | with manufacturers, suppliers and third-parties |
|
IMPORTANT NOTES:  | Confidentiality. BSI will sign a confidentiality agreement with the client before starting any consulting work that involves the use of confidential information. This agreement will protect the client information against unauthorized disclosure (except as required by law). |  | Conflict of Interest. In order to preserve its clients' interests and confidential information, BSI will sometimes decline to accept certain assignments in order to avoid conflict of interest, protect its own integrity and impartiality. We hope you understand our stringent policy and accept our apologies in advance. |

Contact Information- Electronic mail info@BSI-Consulting.com
|
![]()