

| | Health technology is in high and constant demand in developing countries. While some of the need are well justified, often international organizations find itself besieged with requests for excessively sophisticated technologies with little or no evidence of subsequent sustainability. If strategic planning, careful procurement, and aggressive management are essential for industrialized nations, it is even more critical for developing countries. Without proper management and control, instead of improving health conditions, technology will only contribute to poorer and more inefficient health services and widen the gap between the rich and the poor. BSI consultants have accumulated extensive experience in developing countries in three continents as shown below  | Latin America | Argentina |  | Brazil |  |  Colombia Colombia |  | Costa Rica |  | Cuba |  | Ecuador |  | Jamaica |  | Mexico |  | Peru |
|  | Asia |  | Africa | South Africa |
|  | Middle East | Saudi Arabia |
|
BSI consultants have also provided consultative services to major international and bilateral agencies, as well as several non-government organizations as listed below  | The World Bank |  | Inter-American Development Bank - IADB |  | Pan-American Health Organization - PAHO |  | World Health Organization - WHO |  | Carelift International, Philadelphia, PA |  | Orbis International, New York, NY |  | Plymouth Institute, Gladwyne, PA |  | Clapp & Mayne, Rockville, Maryland |  | PSR Consulting Ltd, Helsinki, Finland |  | Booz Allen Hamilton, Beirut, Lebanon |
Because of the vast experience of its talented consultants (click here for some of the international projects), BSI is uniquely positioned to assist technology users to make the right decision for their particular environment in every part of the technology management life cycle. 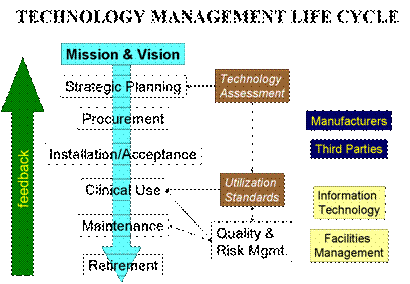
 | Strategic planning  | strategic analysis (strength, weakness, opportunities and threats - SWOT) |  | safety, efficacy and effectiveness assessment reviews |  | potential risks and liabilities exposures evaluations |  | needs assessment (requirements to meet organization mission, vision, goals and objectives) |  | impacts assessment (infrastructure, users, and technical support) |  | cost assessment (total cost of ownership analysis) |  | benefits assessment (clinical, financial, strategic, etc.) |  | return on investment analysis |
|
 | Procurement | technology evaluation |  | product evaluation |  | selection process (bidding, request for proposal, direct negotiation, etc.) |  | purchase agreement |  | alternatives to purchasing (leasing, rental, revenue sharing, etc.) |
|
 | Installation & Acceptance | pre-installation requirements |  | installation verification and validation |  | acceptance testing |  | notification to respective OEM, if required |
|  | Clinical use | user training/inservice and retraining |  | clinical protocol |  | use error monitoring |
|  | Maintenance | servicer training |  | warranty management |  | safety and performance inspections and preventive maintenance |  | revision control |  | service options (internal, OEM, third parties) |  | service records |  | test & measurement equipment calibration |  | service contract review and supervision |
|  | Retirement | retirement and replacement planning |  | replacement criteria |  | notification to respective OEM, if required |  | remarketing or donation |
|  | Quality and Risk Management | FDA general controls against misbranding and adulteration |  | recall program |  | device tracking program |  | complaints management |  | sourcing of alternative parts and subassemblies |  | patient/user incident investigation |  | liability insurance |  | litigation defense |  | compliance to voluntary standards (JCAHO, ISO 9001:2000, etc.) |
|  | Interface | with facility management |  | with information technology |  | with manufacturers, suppliers and third-parties |
|

Contact Information- Electronic mail info@BSI-Consulting.com
|
![]()